Improved Sanitization Methods for Food Processing Facilities
By Mel Barutha, Jackson Burnett, Suzanne Hofford, Ken O'Connor, and Myron Shuler
Presented at the Atlantic Food Development & Processing Exhibition, August 11, 1999
Table of Contents
Introduction
Quat Wiper
Iodine Wiper
Conclusions
Introduction
Contamination issues vary by industry. For microchip makers, particles are the most significant form of contamination. If you have a particle of greater than 0.2 microns, and it gets on a chip when it's being made, you have a chip that will not function and is off quality.
For pharmaceutical manufacturers, bacteria and pyrogens are a greater concern than particles because they contaminate the injectable drug and also create off-quality or unusable products.
Each process has some form of contamination to which exposure makes a product that is unacceptable for sale or consumption. Efforts to eliminate sources of the contamination always result in improved quality and throughput. In many instances, the improved quality also results in improved safety for consumers.
In the food industry, high microbial level contamination can be detrimental to final product quality. Recent product recalls due to unacceptable levels of listeria, E. coli, and salmonella have heightened awareness to this costly and potentially fatal reality.
The most common sources of food contamination are human hands, work surfaces, processing equipment, residuals from chemicals and disinfectants, and cross contamination from other products in the plant.
No longer participants in a low technology industry, increased customer demands for safer, more convenient products manufactured to more stringent quality standards are the expected norm for food processing companies. For example, the new Hazard Analysis and Critical Control Points (HACCP) program has been implemented by the government as a system of contamination control for meat and poultry plants. As pathogens and biological contaminants become resistant to disinfectants, our ability to deal with them needs to become more focused and more flexible. Industries that once produced in uncontrolled environments are now finding themselves legally responsible for activities not addressed previously. Uncontrolled environments in the food industry are increasingly moving to controlled environments, cleanrooms, and ultimately aseptic areas. Products and protocols for process control are becoming more complex. Training an ever-changing employee base makes effective implementation more problematic. In the future, high-tech solutions for high-tech areas will need to be simpler.
For years, product quality was assured through vigorous sanitization methods. Many sanitizing agents and methods were employed. Some included general spraying of equipment by night cleaning crews and wipe down of surfaces between shifts coupled with general housekeeping methods throughout the processing facility.
But it is well known that the best sanitation procedures are those that are simple and have the least chance of variation. Cleaning that is performed by hand can result in human error that sometimes plays a significant role in the failure of existing sanitization methods. Outgoing product quality levels depend on the successful completion of assigned duties and responsibilities by cleaning and sanitizing personnel.
For the most part current cleaning methods are complex. The associates performing this work must first clean and sanitize themselves prior to donning required garments and safety equipment. This is a very critical activity as we have already learned from the cleanroom processing industries since the most likely source of product contamination is exposure to individuals working in process areas.
Because sanitizing chemicals are provided in bulk packaging and, in many instances, in concentrated form, personnel performing cleaning operations are expected to measure out prescribed amounts of the sanitizing material for their daily use. In addition to measuring materials, the associates are required to dilute chemicals to the proper use dilution and mix the resultant solution to insure the proper level of activity. As you might expect, the possibility for errors increases especially when production levels ramp up and product changeovers are more frequent.
Additional challenges to be addressed include proper application of sanitizing solutions to irregular surfaces or to complex assemblies with several layers of internal surfaces that must be thoroughly sanitized. The application method is many times left to the discretion of the cleaning associates. Generally assumed to be a simple choice, the decision to spray or wipe on sanitizers is not always specified by the chemical manufacturer. As a result, the food processing company --- specifically the cleaning personnel --- makes the call. Little assistance is provided by the chemical supplier in determining the proper amount of solution to be applied to the surfaces to be sanitized. As a result, the interpretation of the proper sanitization will vary from employee to employee. The process will not be reproducible and the level of microbial reduction unpredictable.
Efficacy data to validate the sanitizing process is often not available, requiring that the data be developed by food processors. If time is not taken to develop validated methods, the resultant sanitizing process will be unreliable and a wasteful endeavor.
Effective sanitizing processes are based on the assumption that the surface has been thoroughly cleaned and decontaminated of food residues, meat residues and waxy coatings of fat. It is important that protocols address methods for proper cleaning of equipment, utensils and other food contacting surfaces. Since many raw foods are not completely soluble in aqueous solutions, cleaning must be performed with systems that will solubilize fats and proteins, but be free rinsing prior to application of the sanitizing solutions. The cleaning systems must be approved for food applications and not limit the efficacy of the sanitizer.
To reduce the possibility of error, food processors need to employ simple and convenient cleaning and sanitizing methods that require minimal effort. As the food industry moves to adopt increased quality standards, the ideal methods will be readily integrated into good manufacturing practices systems of reproducibility, efficacy validation and lot to lot traceability.
Drawing once again from the model in other industries, the implementation of improved, simplified methods while addressing bona fide safety issues afford as well, certain competitive advantages. Through simplification of processes, the time required for cleaning and sanitization is reduced resulting in decreased labor costs. Improved sanitation methods will contribute to reduced product microbial levels with potential increases in product shelf life, taste and appearance, and a reduction in waste.
As increased demands are placed on manufacturers to minimize the exposure of consumers to toxic levels of microbial contamination, it will be necessary to re-define current methods of food manufacturing. More companies will implement good manufacturing methods and in some instances consider aseptic processing. In fact some meat products will soon be exposed to low levels of gamma radiation in an attempt to further decrease and control pathogens in raw meat products. In this paper we will discuss two new sanitizing systems that can be readily adopted in all food processing applications. The improved methods can be implemented with few changes to existing systems at minimal cost, but with dramatic reductions in foodborne pathogens and increased control of product quality.
Quat Wiper
The first system is for the sanitization of food contacting surfaces, other than worker hands. In this system, a nonwoven wiper fabric is coated with a metered amount of a solution of quaternary ammonium compounds (quat). Following the coating process, the wiper substrate is dried, cut to size and packaged. The quaternary compounds incorporated into the wiper substrate are widely recognized as safe and efficacious for sanitizing food-contacting surfaces when used in concentrations less than 200 ppm. In this system the concentration ranges from 140 to 180 ppm.

A dual quat system was selected because it is more effective than either quat when used alone. There is a synergistic effect when used in combination, because a more optimum balance between hydrophilicty and lipophilicity is established with a dual system. As a result a dual quat system is more effective at traversing cell wall barriers, allowing for greater germicidal efficacy [1].
The wider variety of alkyl groups provides a greater diversity of microbial activity with yeast and fungi more sensitive to C12, gram-positive bacteria to C14 and gram-negative bacteria more sensitive to C16 quaternary ammonium compounds [2].
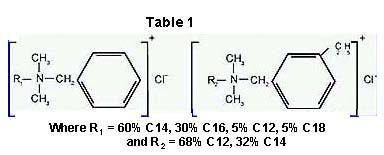
The dual quat system in this improved method of sanitization consists of a 50/50 mixture of N-Alkyl (60% C14, 30% C16, 5% C12, 5% C18) dimethyl benzyl ammonium chlorides and N-Alkyl (68% C12, 32% C14) dimethyl ethyl benzyl ammonium chlorides as the active ingredients of the surface sanitizing wiper. Chemical structures for the dual quat system are provided in Table 1. Inert ingredients include propylene glycol as the diluent along with a wetting agent and a Turquoise pigment. The diluent is required to insure uniform coating on the wiper and, ultimately, reliable release from the nonwoven substrate during use. The wetting agent works in concert with the propylene glycol, insuring that the sanitizing solution uniformly coats the irregularities of the surfaces to be sanitized. The additional wetting agent augments the surfactant effects of the higher molecular weight quats to insure the thorough wetting of hydrophobic surfaces made from polypropylene, polyethylene and polyester plastics.
A couple of very valid questions should be asked. How are the concentrations of the solutions applied to each towel verified? And how can you be sure that you are getting the necessary amount of chemical on the towels to perform the task? As with any process, quality control is of the utmost importance. The method of verifying the concentration of the initial solution applied to each towel and of the active solution on the towel is titration. This method is an accurate way to determine the concentrations of the active ingredients present in the solutions before application on the towels and once applied to the towels. Titration is performed by reaction with sodium tetraphenylboron in the presence of methyl orange as the indicator [3] . In the titration, the methyl orange changes from a green color to milky, amber red at the equivalence point.
The food surface contact towel is activated by placing the towel in one gallon of water - hot or cold. Due to the presence of the Turquoise pigment, the solution becomes a green color, indicating that the water is an active quaternary ammonium compound solution that contains less than 200 ppm active quat. This solution may be used for up to eight (8) hours on surfaces that have been thoroughly cleaned prior to sanitization. It is important to note that the quat wiper should be used to sanitize only surfaces that have been thoroughly cleaned. Introduction of organic debris from meat cutting or other gross food particles will limit the effectiveness of the quat system and greatly reduce usable life of the activated solution.
During the manufacture of the quat wiper, the fabric is immersed in a quaternary ammonium solution bath that contains 250 ± 50 ppm quaternary compound. By controlling the add-on weight of the solution, the resultant surface sanitizing towel will contain 160 ± 20 ppm quaternary compound.
Testing of the effectiveness of the quat wiper on food contacting surfaces indicated that the concentration of the activated solution is effective in reducing microbial levels on food contacting surfaces. Table 2 summarizes the testing conducted to date on actual processing surfaces used in the production of spices and ready to eat food products. Results are reported as aerobic plate counts ( CFU --- colony forming units ) taken using sterile swabs from a 2 inch by 2 inch square area of each test surface. The sanitization process was accomplished by using the wiper to thoroughly wet the surface with the activated solution, which was allowed to remain in place for at least one minute. Following exposure, excess solution was wiped up and the surface was allowed to dry prior to swabbing with sterile swabs.
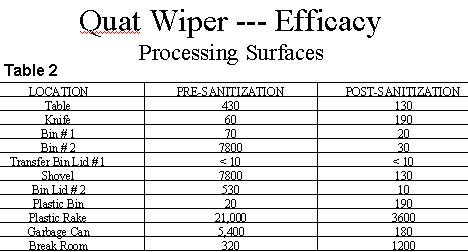
None of the samples contained Staphylococcus aureus, Salmonella or Escherichia coli CFU's. With the exception of two results, significant reduction in plate counts were observed. The quat wiper exhibited the greatest effect on surfaces with the highest microbial load prior to sanitization. The increase observed on the Break Room surface was attributed to experimental error, whereby the 2 inch by 2 inch sampling template was exposed to an unsanitized area.
Similar favorable results were observed on the surfaces of gloved and ungloved hands as depicted in Tables 3 and 4.
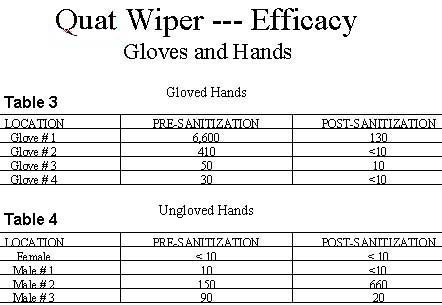
As indicated previously, ungloved hands are a potential source of contamination in most food processing environments. However, the hands of the subjects in this test even when ungloved were found to be relatively low in microbial contamination.
Similar favorable sanitizing results were observed on surfaces found in the packaging area and spice processing equipment. Tables 5 and 6 summarize the results of this testing. Once again, the results are more dramatic on sanitizing surfaces with the higher levels of microbial contamination.
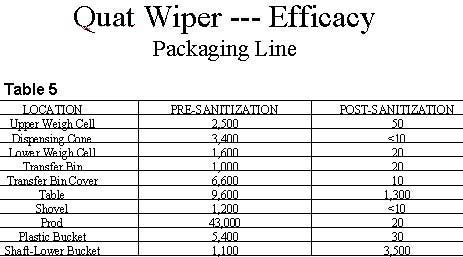
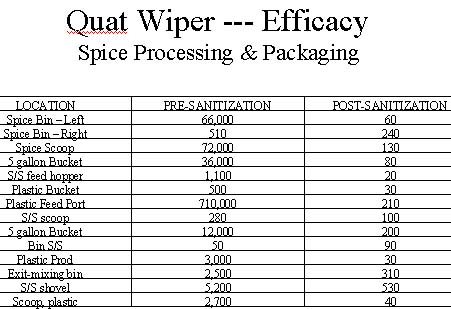
Quaternary ammonium compounds are potent, broad-spectrum disinfectants. When used as active ingredients in formulations such as hard surface disinfectants, sanitizers and/or certain types of water treatment formulations, quaternary ammonium compounds have been found to provide superior biocidal action against a broad spectrum of microbial organisms such as: bacteria, fungi, viruses and algae. Quaternary ammonium compounds especially the dual quat systems deliver potent germicidal action even in heavy organic soil loads. Based on the test data, one can conclude that the quat wiper method of delivering quaternary ammonium based disinfectants is a safe, simple and effective approach for sanitizing food processing surfaces and equipment. Since the testing was conducted on surfaces sanitized with a single application of the quat wiper system, it can be expected that multiple applications of the quaternary ammonium sanitizer will provide further reductions in surface microbial levels.
Iodine Wiper
The iodine wiper represents a new technology for the application of antimicrobial materials to the hands of food processing / food handling personnel. Essentially, the wiper is a handwipe towel that is comprised of a 5% Povidone-Iodine solution (PVP) coated onto a 100% polypropylene nonwoven wiper substrate. The PVP wiper is designed to provide a uniform amount of sanitizer to the surface of the hand. In its present embodiment, the treated wiper can be used to reduce microbial levels on worker hands via a 15 second wipe on hands that are dry or pre-wetted.
The PVP solution is mechanically applied using processes similar to those employed in the preparation of the quat wiper. The 5% povidone-iodine solution is lightly applied onto the surface of the towel substrate. The 5% PVP solution contains 0.5% active iodine. By controlling add-on weights, the resultant Iodine Wiper contains 750 + 250 ppm (0.075 + 0.025 %) iodine.
Titration for active iodine involves reaction with lactic acid and potassium iodide in the presence of sodium thiosulfate. Addition of lactic acid and potassium iodide to the iodine solution under test imparts a blue coloration to the solution. The solution changes from blue to a clear liquid at the equivalence point upon the addition of a standard sodium thiosulfate solution [4].
For many years, PVP iodine solutions have been recognized as effective antimicrobial agents for use on human skin. Specifically, a FDA Monograph [5] issued in 1994 allows the use of 5 to 10% PVP iodine solutions as an antiseptic handwash for healthcare personnel.
PVP iodine has broad-spectrum activity - against bacteria, fungi, viruses, protozoa cysts and spores. It has been used as a general antiseptic in the treatment of skin infections, cuts, etc. It is the main component in the popular Betadine solution. PVP iodine is also the antimicrobial agent used in physicians' scrub kits.
The antimicrobial properties of PVP iodine coupled with a low toxicity to humans suggests that the use of this compound as part of a quality control regimen in food processing facilities will contribute to the reduction of in-plant microbial levels. In particular use as an antiseptic hand wipe will afford better protection against contamination from the hands of food processing personnel.
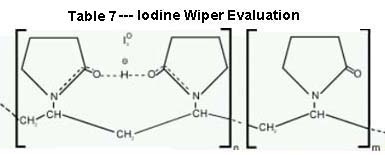
PVP iodine is a complex molecule in which iodine is bound to the carrier molecule, povidone. The complex, PVP Iodine, slowly releases inorganic iodine when in contact with the skin. Only a small amount of iodine is released at any one time, giving PVP iodine a lower irritant potential and longer duration of microbicidal action than conventional iodine solutions. The chemical structure of PVP Iodine is provided in Table 7.
The iodine wiper was evaluated under actual use conditions to determine if it would be effective in reducing microbial levels on human hands. As discussed previously, contamination from hands is one of the most likely sources of food microbial levels. The debate continues whether it is possible to thoroughly decontaminate or disinfect human skin. As a result, current food code regulations require that workers handling food wear gloves. Gloves become of increasing importance when workers either incorrectly wash their hands or avoid the practice altogether. Table 8 summarizes the results of a study on the handwashing habits of people using public washrooms sponsored by the Allegheny County, PA Department of Health.

With this level of contaminated hands, it seemed reasonable to consider the iodine wiper as a method to augment handwashing, especially when ungloved hands are used to handle food or as a backup system in the event of glove failure.
To test the iodine wiper, a group of subject volunteers who refrained from using topical antimicrobials for at least one week prior to testing were selected. The effectiveness of the iodine wiper as an antimicrobial agent was measured by comparing the number of marker bacteria --- Serratia marcescens --- recovered from artificially contaminated hands after use of the wiper to the number of bacteria recovered from contaminated untreated hands. The effectiveness of the wiper was measured after the 1st, 3rd, 5th, 7th and 10th contaminations. All contaminations/treatments occurred in the same day during a two hour period.
The iodine wiper treatment of each test subject followed the product instructions for use directions to " Rinse hands with water, then dry hands for 15 seconds using the iodine treated towel, wiping all skin surfaces on the hands including fingers and cuticles". The results of this experimentation is reported in Table 9.
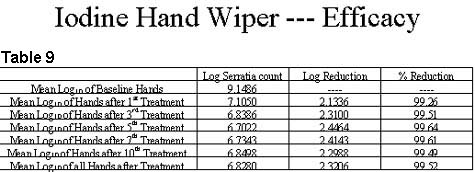
Review of the results of this testing indicates that the reductions in bacterial counts obtained through the use of the iodine wiper are similar to the reductions achieved through the use of iodine-based liquid hand wash preparations.
Conclusions
We have reviewed opportunities to increase contamination control in food processing facilities. Particularly in light of increased public concern and a heightened regulatory environment, the most efficacious sanitation methods will be those that are viewed as being simple, convenient, reproducible, safe, and yet cost effective. This paper has discussed two methods, which can be implemented into existing control programs with dramatic reductions in microbial levels. We provided data to support our claims of microbial reduction measures under actual use conditions. Because both wiper systems may be used in a number of process, storage and packaging areas, implementation may be done throughout the facility or selectively at critical control points. Use of the iodine wiper should be considered in all restrooms, gowning and other break areas.
Literature Cited
- 1. Disinfection, Sterilization, and Preservation , fourth edition, Seymour S. Block, page 228.
- 2. Disinfection, Sterilization, and Preservation , fourth edition, Seymour S. Block, page 238.
- 3. Test Method # 3043, LaMotte Company, Chestertown, Maryland.
- 4. Standard Methods for the examination of Water and Wastewater, seventeenth edition, pages 4-106 to 4-108.
- 5. 21 Code of Federal Regulations, Parts 333 and 369, Tentative Final Monograph for Health-Care Antiseptic Drug Products; Proposed Rule, June 17, 1994.
For information about products mentioned in the article, contact Contec, Inc., P.O. Box 530, Spartanburg, SC 29304. Tel: 864-503-8333; Fax: 864-503-8444.
